
Company Overview NASDAQ: ACST April 2024

Forward Looking Statements Statements in this presentation that are not statements of historical or current fact constitute "forward-looking statements" within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, as amended, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, and "forward-looking information" within the meaning of Canadian securities laws (collectively, "forward-looking statements"). Such forward looking statements involve known and unknown risks, uncertainties, and other factors that could cause the actual results of Acasti to be materially different from historical results or from any future results expressed or implied by such forward-looking statements. In addition to statements which explicitly describe such risks and uncertainties, readers are urged to consider statements containing the terms "believes," "belief," "expects," "intends," "anticipates," "estimates", "potential," "should," "may," "will," "plans," "continue", "targeted" or other similar expressions to be uncertain and forward-looking. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this presentation. The forward-looking statements in this presentation, including statements regarding the Company's anticipated cash runway, the timing of the planned initiation of the Company's STRIVE-ON trial and the resulting data readout, the timing of the Company's anticipated NDA submission with the FDA, GTX-104's potential to bring enhanced treatment options to patients suffering from aSAH, any future patent filings made by the Company for new developments and the anticipated trial design of STRIVE-ON are based upon Acasti's current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, including, without limitation: (i) the success and timing of regulatory submissions of the planned Phase 3 safety trial for GTX-104; (ii) regulatory requirements or developments and the outcome and timing of the proposed NDA application for GTX-104; (iii) changes to clinical trial designs and regulatory pathways; (iv) legislative, regulatory, political and economic developments; (v) actual costs associated with Acasti's clinical trials as compared to management's current expectations; and (vi) the other risk factors identified in the Company's Annual Report on Form 10-K for the year ended March 31, 2023. The foregoing list of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors detailed in documents that have been and are filed by Acasti from time to time with the Securities and Exchange Commission and Canadian securities regulators. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Acasti undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by applicable securities laws.

Late-stage, biopharma company poised to disrupt Standard of Care (SoC) in treatment of aneurysmal Subarachnoid Hemorrhage (aSAH) Summary GTX-104 – novel intravenous nimodipine – well positioned to solve oral challenges and potentially displace it as the SoC Nimodipine is the SoC and clinically de-risked however, significant unmet needs with its only available dosage form (oral) Regulatory pathway to NDA filing is de-risked requiring one safety trial; compelling safety data in over 160 subjects Pivotal Phase 3 STRIVE-ON safety trial began enrolling patients in 4Q 2023 $300M+ annual US market opportunity with ODD and strong patent estate Reported cash anticipated to fund STRIVE-ON trial and potential NDA filing in 1H 2025; cash runway into 2Q 2026 Sources: Fletcher Spaght market research report. Dates based on calendar year.
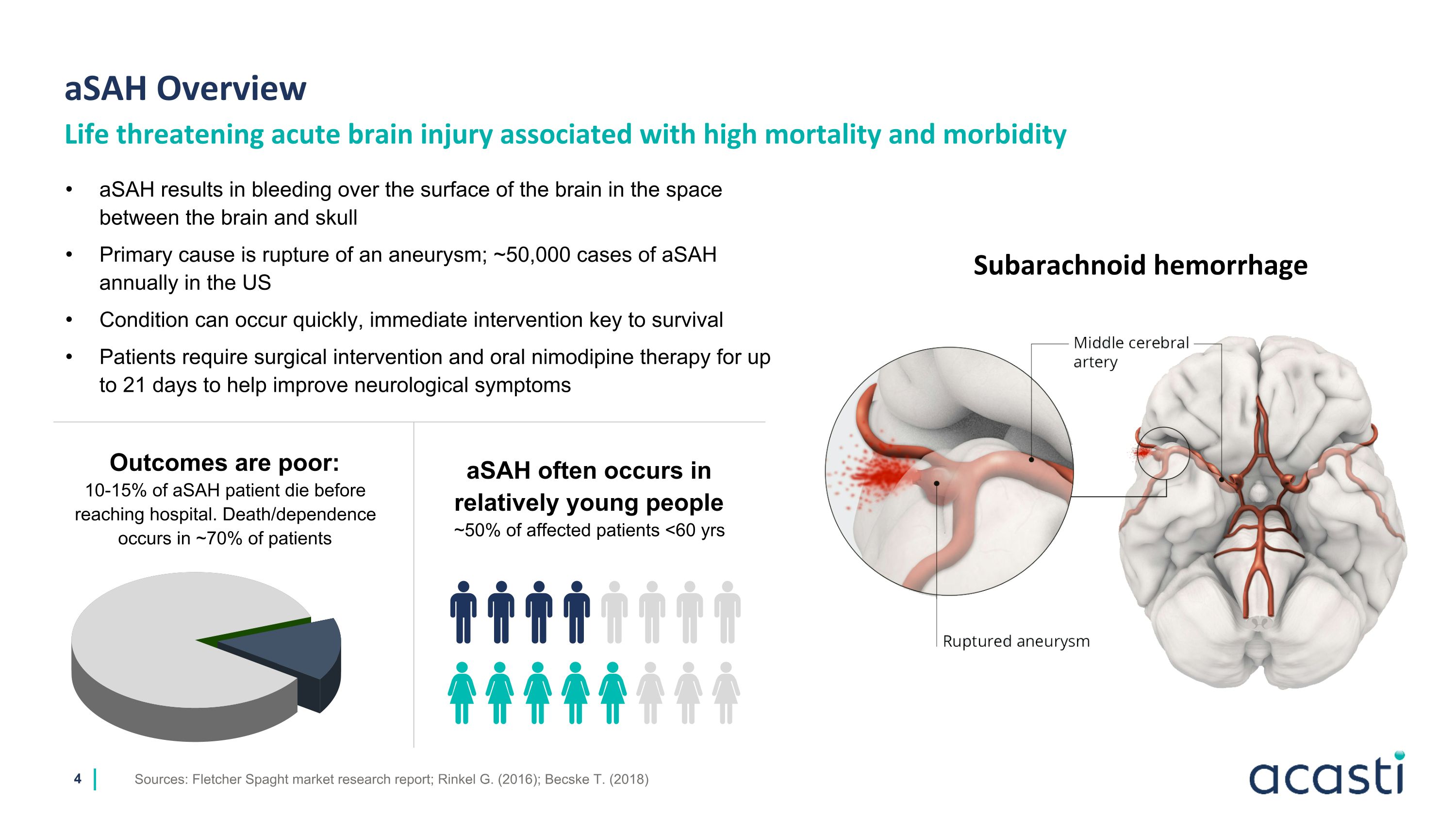
Sources: Fletcher Spaght market research report; Rinkel G. (2016); Becske T. (2018) Life threatening acute brain injury associated with high mortality and morbidity aSAH Overview Subarachnoid hemorrhage aSAH results in bleeding over the surface of the brain in the space between the brain and skull Primary cause is rupture of an aneurysm; ~50,000 cases of aSAH annually in the US Condition can occur quickly, immediate intervention key to survival Patients require surgical intervention and oral nimodipine therapy for up to 21 days to help improve neurological symptoms Outcomes are poor: 10-15% of aSAH patient die before reaching hospital. Death/dependence occurs in ~70% of patients aSAH often occurs in relatively young people ~50% of affected patients <60 yrs

Soppi V. (2007); Abboud T. (2015); Connolly ES. (2012) Substantial barriers to administering oral nimodipine aSAH Current Standard of Care Current limitations Many aSAH patients are unconscious, cannot swallow oral medication - delivery via nasogastric tube often necessary, resulting in significant dose variability Highly variable absorption and first pass effect (CYP3A4 drugs) - leading to unpredictable hypotension Narrow and burdensome administration window Dose-limiting side-effects such as hypotension due to unpredictable absorption, low bioavailability and high first-pass metabolism Side-effects Dose reduction or discontinuation of nimodipine is common, potentially leading to poor outcomes Reduction of dose Oral Nimodipine 60 mg (two large 30 mg capsules) every four hours
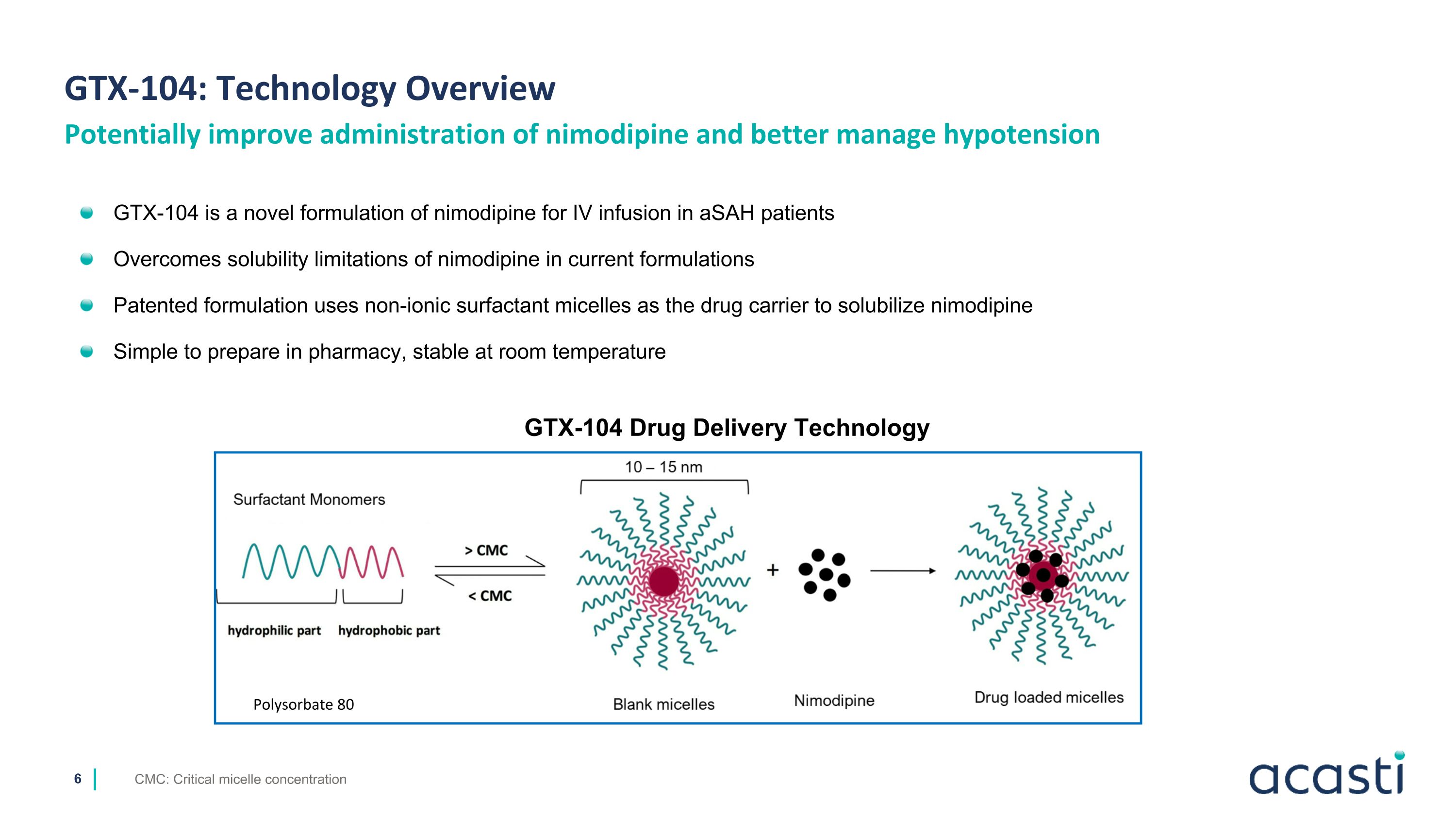
Potentially improve administration of nimodipine and better manage hypotension GTX-104 is a novel formulation of nimodipine for IV infusion in aSAH patients Overcomes solubility limitations of nimodipine in current formulations Patented formulation uses non-ionic surfactant micelles as the drug carrier to solubilize nimodipine Simple to prepare in pharmacy, stable at room temperature GTX-104: Technology Overview GTX-104 Drug Delivery Technology Polysorbate 80 CMC: Critical micelle concentration
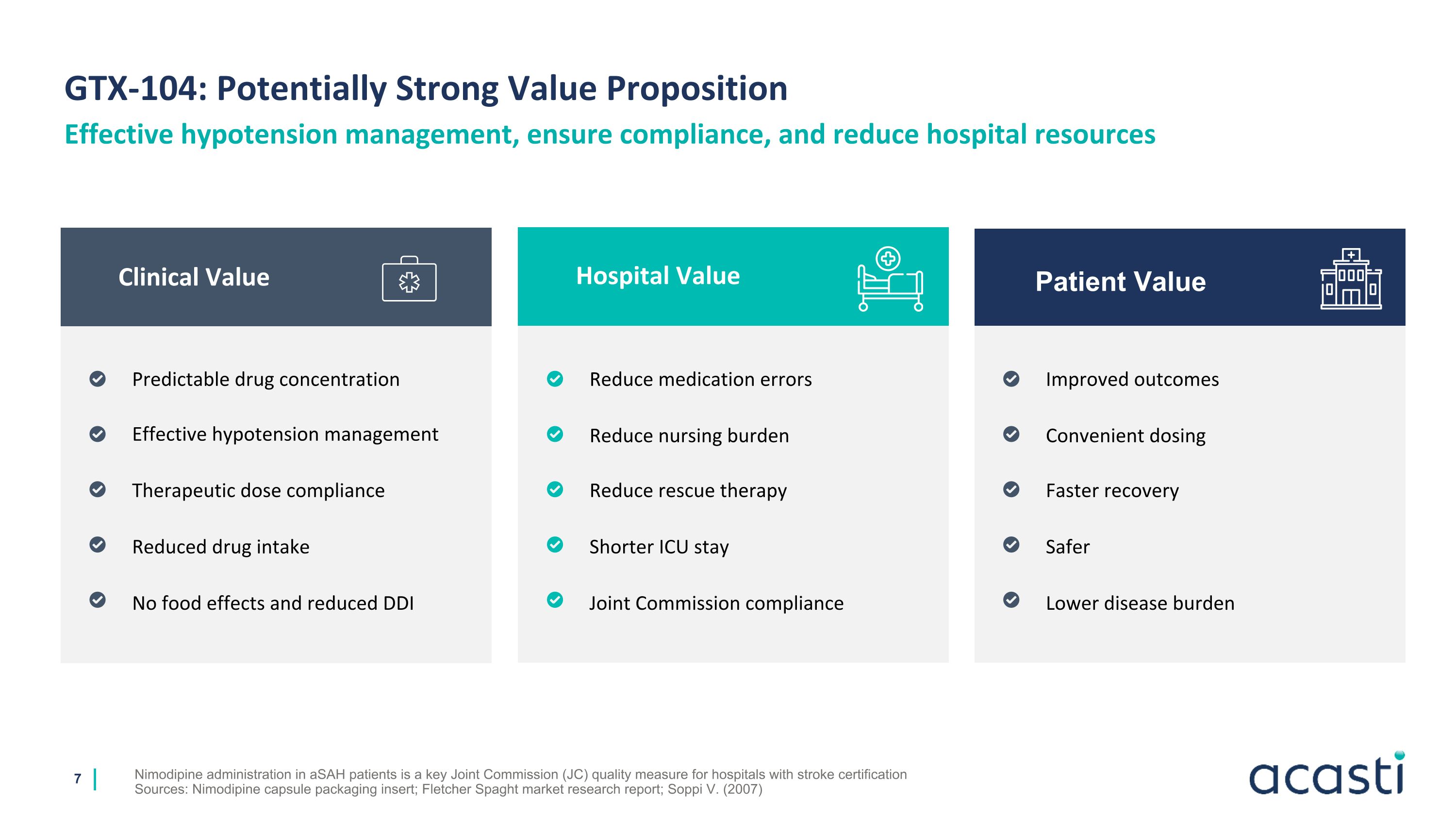
GTX-104: Potentially Strong Value Proposition Nimodipine administration in aSAH patients is a key Joint Commission (JC) quality measure for hospitals with stroke certification Sources: Nimodipine capsule packaging insert; Fletcher Spaght market research report; Soppi V. (2007) Effective hypotension management, ensure compliance, and reduce hospital resources Predictable drug concentration Effective hypotension management Therapeutic dose compliance Reduced drug intake No food effects and reduced DDI Clinical Value Patient Value Hospital Value Reduce medication errors Reduce nursing burden Reduce rescue therapy Shorter ICU stay Joint Commission compliance Improved outcomes Convenient dosing Faster recovery Safer Lower disease burden
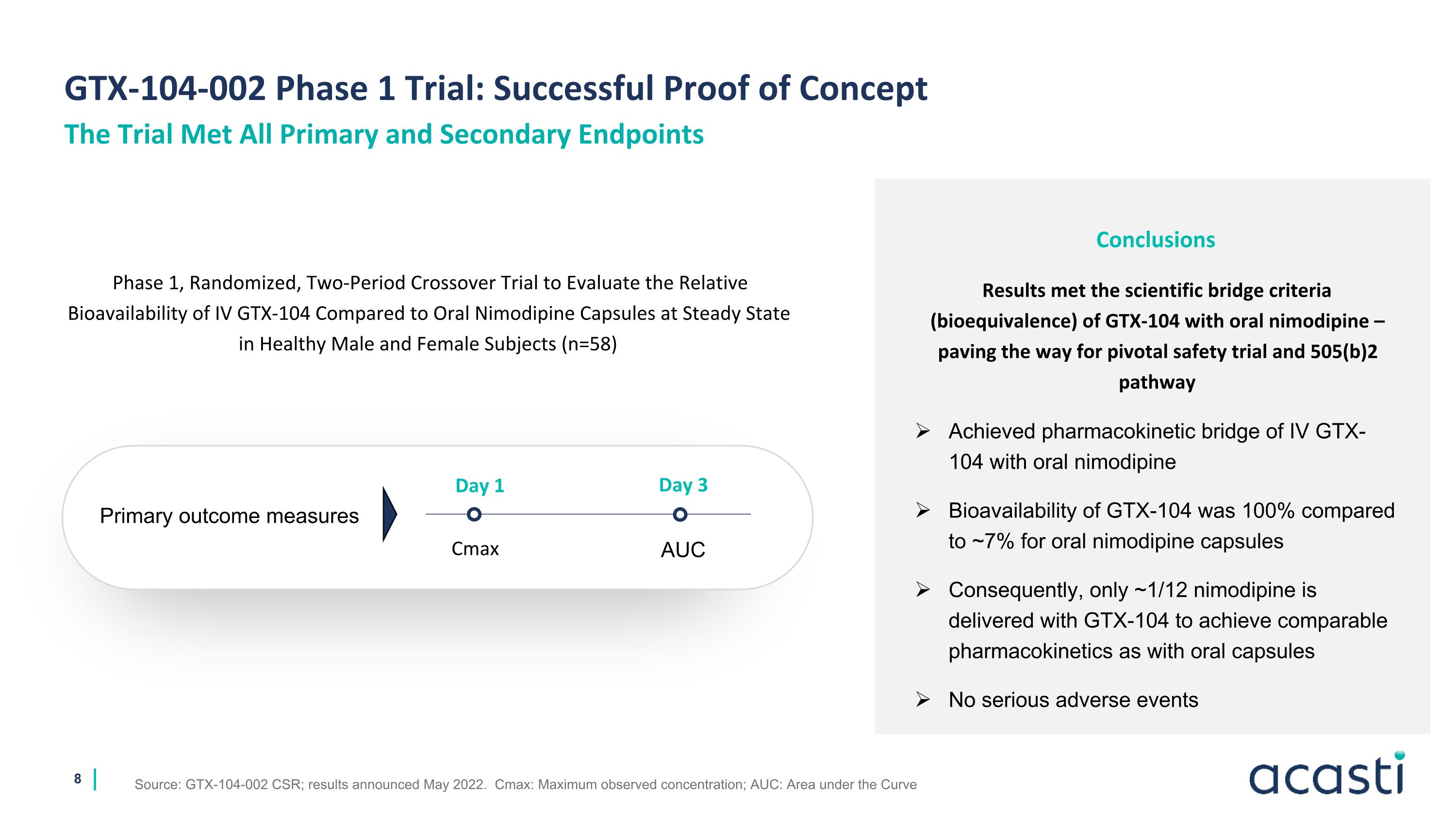
GTX-104-002 Phase 1 Trial: Successful Proof of Concept Source: GTX-104-002 CSR; results announced May 2022. Cmax: Maximum observed concentration; AUC: Area under the Curve The Trial Met All Primary and Secondary Endpoints Conclusions Results met the scientific bridge criteria (bioequivalence) of GTX-104 with oral nimodipine – paving the way for pivotal safety trial and 505(b)2 pathway Achieved pharmacokinetic bridge of IV GTX-104 with oral nimodipine Bioavailability of GTX-104 was 100% compared to ~7% for oral nimodipine capsules Consequently, only ~1/12 nimodipine is delivered with GTX-104 to achieve comparable pharmacokinetics as with oral capsules No serious adverse events Phase 1, Randomized, Two-Period Crossover Trial to Evaluate the Relative Bioavailability of IV GTX-104 Compared to Oral Nimodipine Capsules at Steady State in Healthy Male and Female Subjects (n=58) Primary outcome measures Day 1 Day 3 AUC Cmax
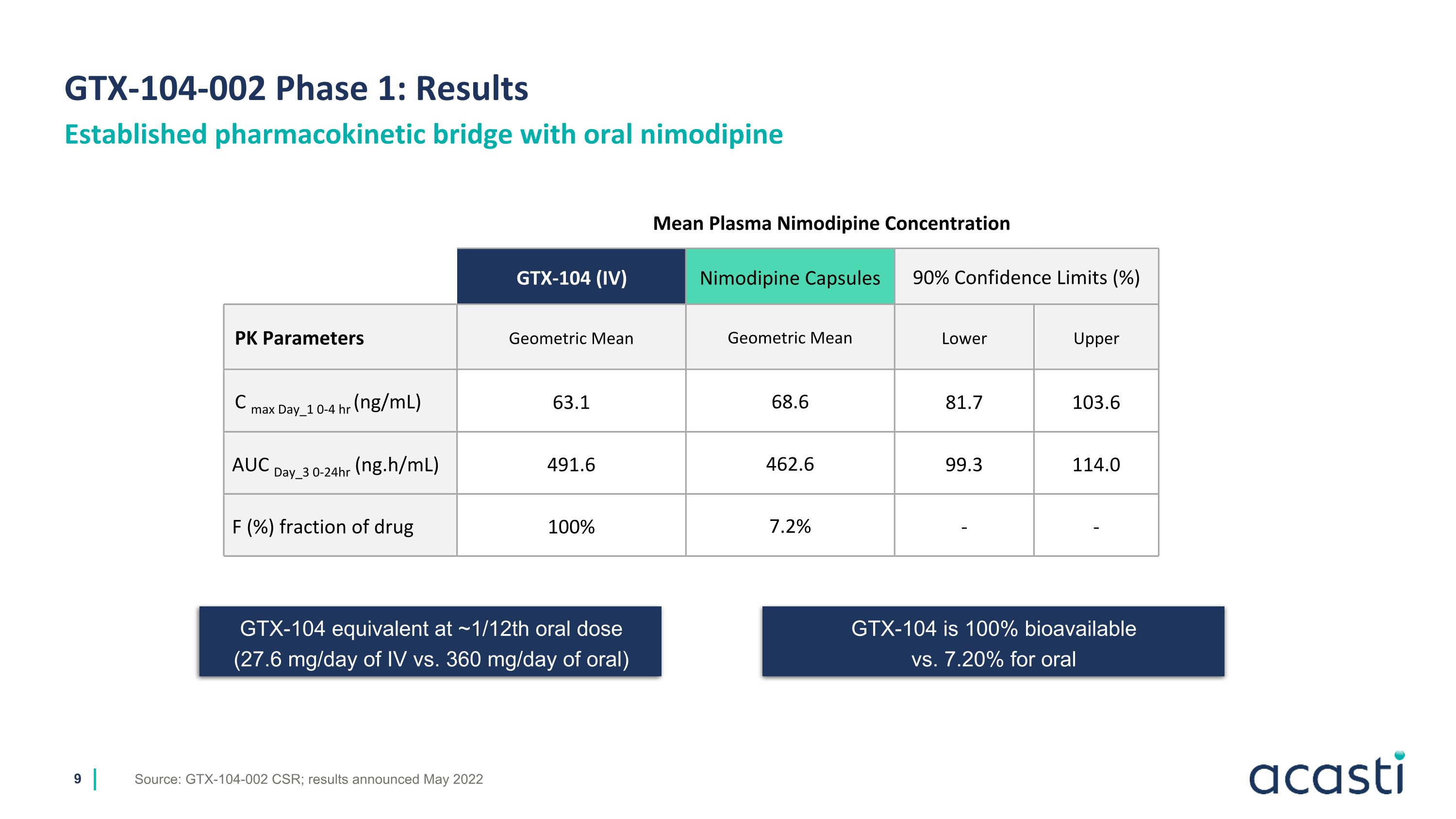
GTX-104-002 Phase 1: Results Source: GTX-104-002 CSR; results announced May 2022 Established pharmacokinetic bridge with oral nimodipine GTX-104 is 100% bioavailable vs. 7.20% for oral Mean Plasma Nimodipine Concentration GTX-104 (IV) Nimodipine Capsules 90% Confidence Limits (%) PK Parameters Geometric Mean Geometric Mean Lower Upper C max Day_1 0-4 hr (ng/mL) 63.1 68.6 81.7 103.6 AUC Day_3 0-24hr (ng.h/mL) 491.6 462.6 99.3 114.0 F (%) fraction of drug 100% 7.2% - - GTX-104 equivalent at ~1/12th oral dose (27.6 mg/day of IV vs. 360 mg/day of oral)
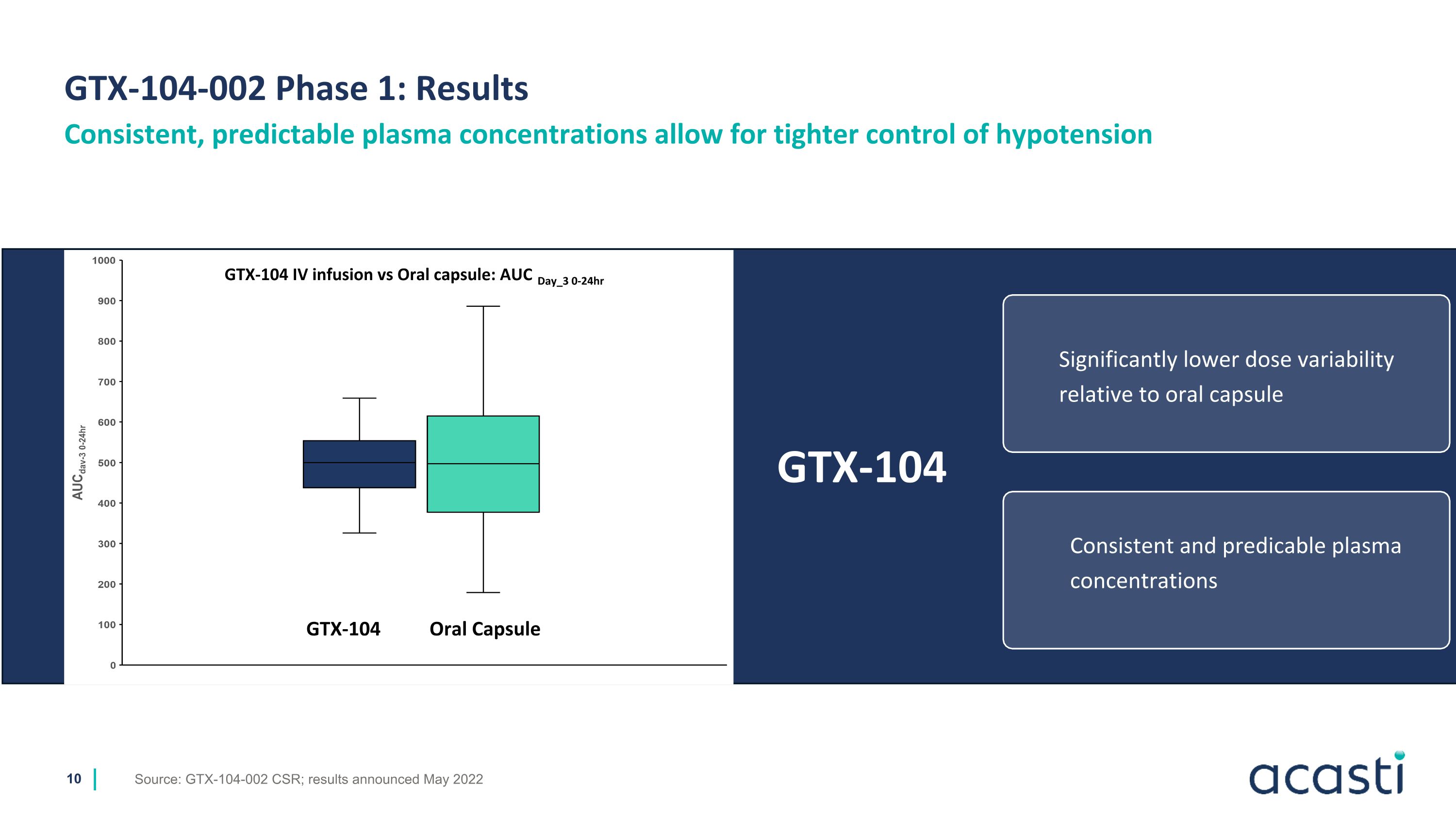
GTX-104-002 Phase 1: Results Source: GTX-104-002 CSR; results announced May 2022 Consistent, predictable plasma concentrations allow for tighter control of hypotension 37% 15% 37% 11% Significantly lower dose variability relative to oral capsule Consistent and predicable plasma concentrations GTX-104 GTX-104 IV infusion vs Oral capsule: AUC Day_3 0-24hr GTX-104 Oral Capsule
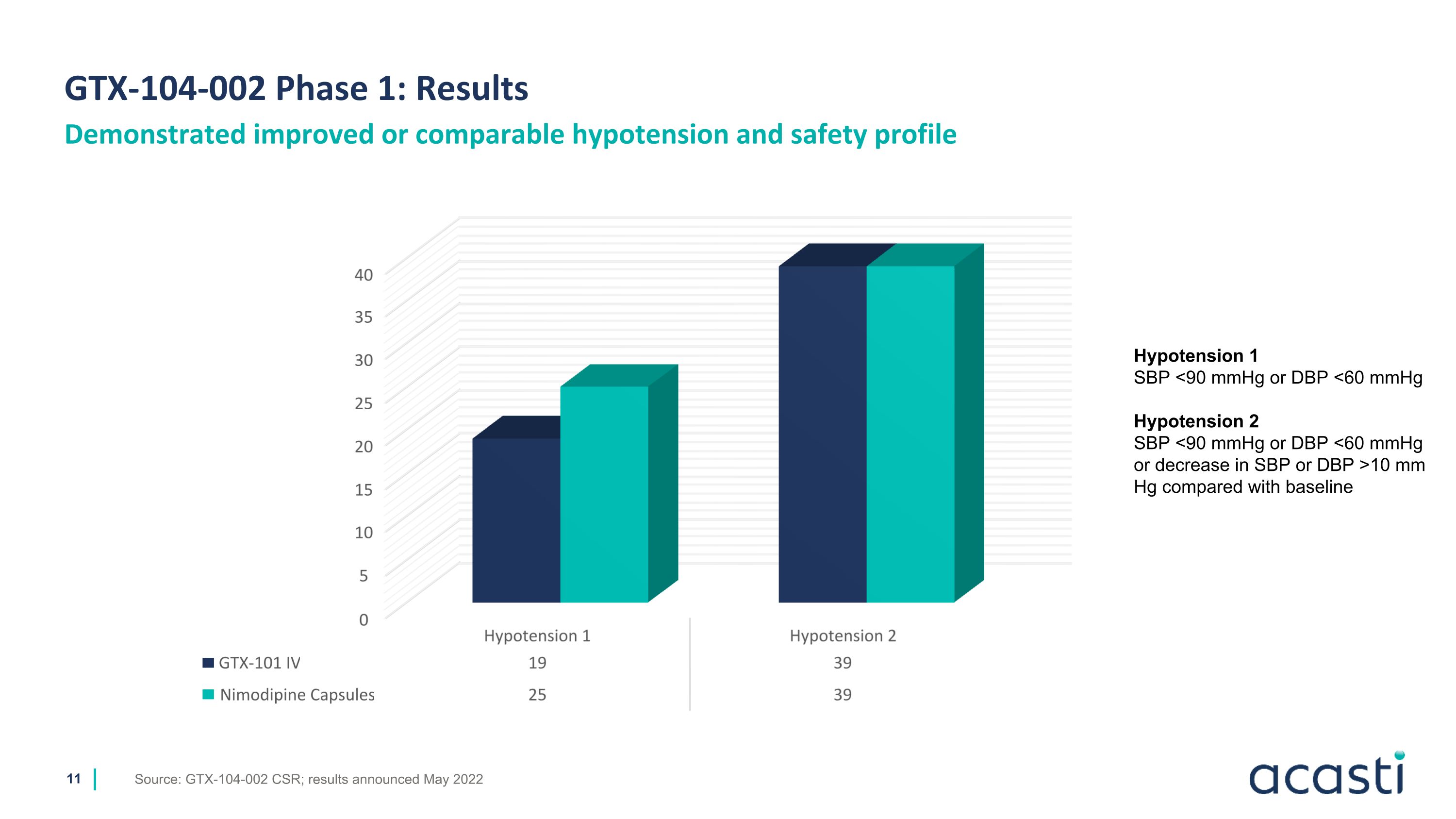
GTX-104-002 Phase 1: Results Demonstrated improved or comparable hypotension and safety profile Source: GTX-104-002 CSR; results announced May 2022 Hypotension 1 SBP <90 mmHg or DBP <60 mmHg Hypotension 2 SBP <90 mmHg or DBP <60 mmHg or decrease in SBP or DBP >10 mm Hg compared with baseline
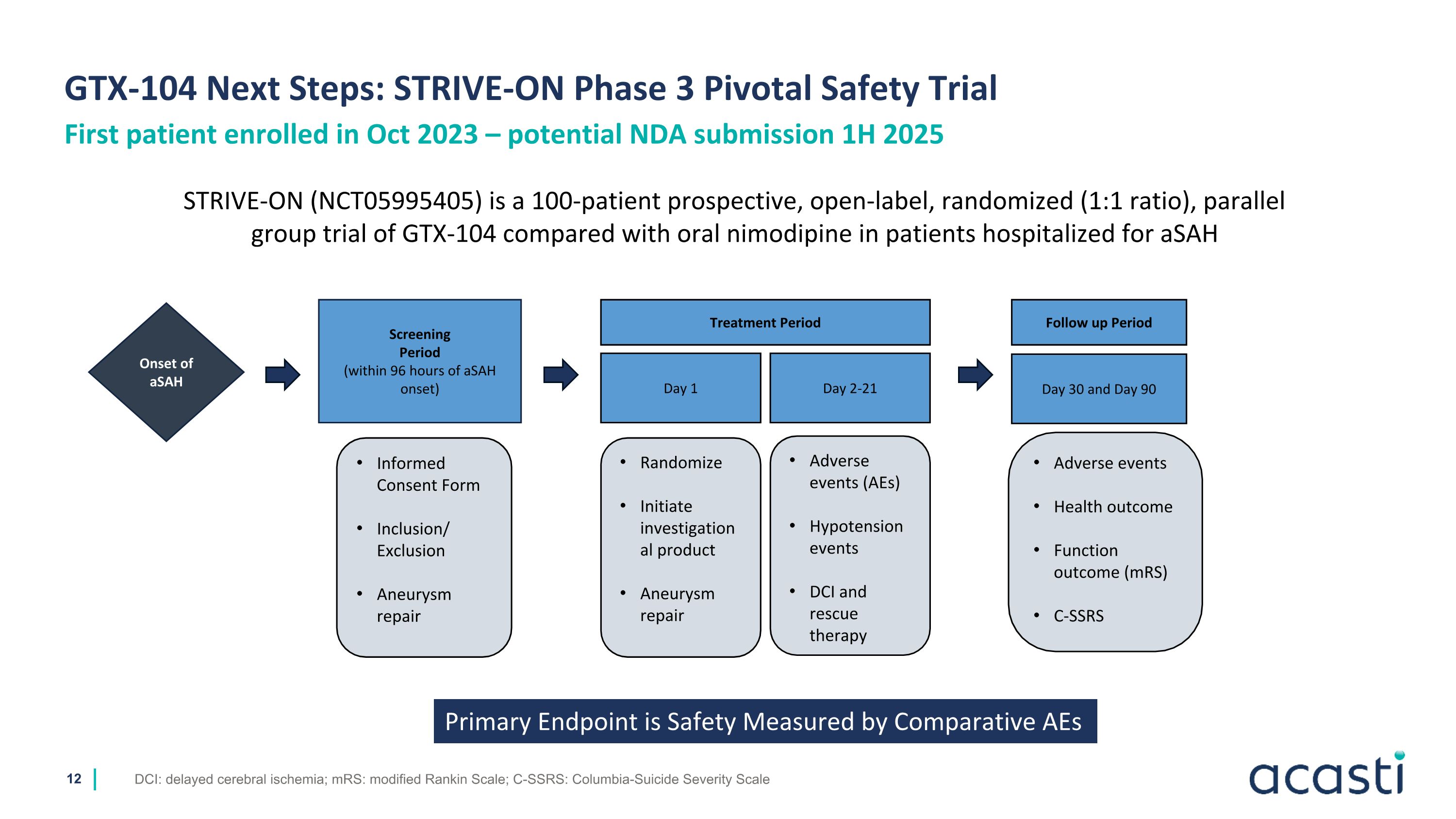
GTX-104 Next Steps: STRIVE-ON Phase 3 Pivotal Safety Trial DCI: delayed cerebral ischemia; mRS: modified Rankin Scale; C-SSRS: Columbia-Suicide Severity Scale First patient enrolled in Oct 2023 – potential NDA submission 1H 2025 STRIVE-ON (NCT05995405) is a 100-patient prospective, open-label, randomized (1:1 ratio), parallel group trial of GTX-104 compared with oral nimodipine in patients hospitalized for aSAH Screening Period (within 96 hours of aSAH onset) Day 1 Treatment Period Day 2-21 Onset of aSAH Informed Consent Form Inclusion/ Exclusion Aneurysm repair Randomize Initiate investigational product Aneurysm repair Adverse events (AEs) Hypotension events DCI and rescue therapy Follow up Period Day 30 and Day 90 Adverse events Health outcome Function outcome (mRS) C-SSRS Primary Endpoint is Safety Measured by Comparative AEs
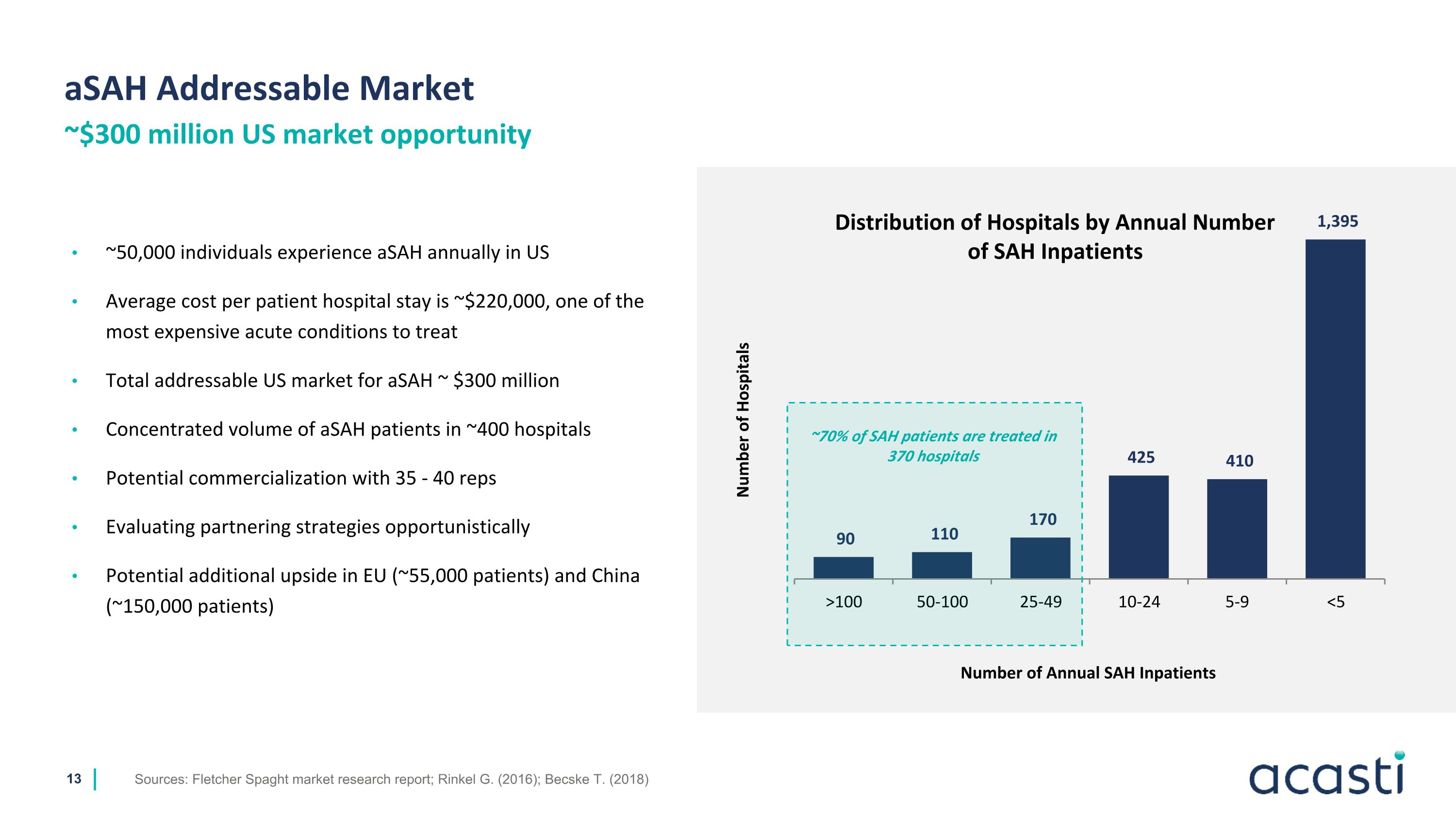
aSAH Addressable Market Sources: Fletcher Spaght market research report; Rinkel G. (2016); Becske T. (2018) ~$300 million US market opportunity ~50,000 individuals experience aSAH annually in US Average cost per patient hospital stay is ~$220,000, one of the most expensive acute conditions to treat Total addressable US market for aSAH ~ $300 million Concentrated volume of aSAH patients in ~400 hospitals Potential commercialization with 35 - 40 reps Evaluating partnering strategies opportunistically Potential additional upside in EU (~55,000 patients) and China (~150,000 patients) Number of Hospitals ~70% of SAH patients are treated in 370 hospitals Distribution of Hospitals by Annual Number of SAH Inpatients
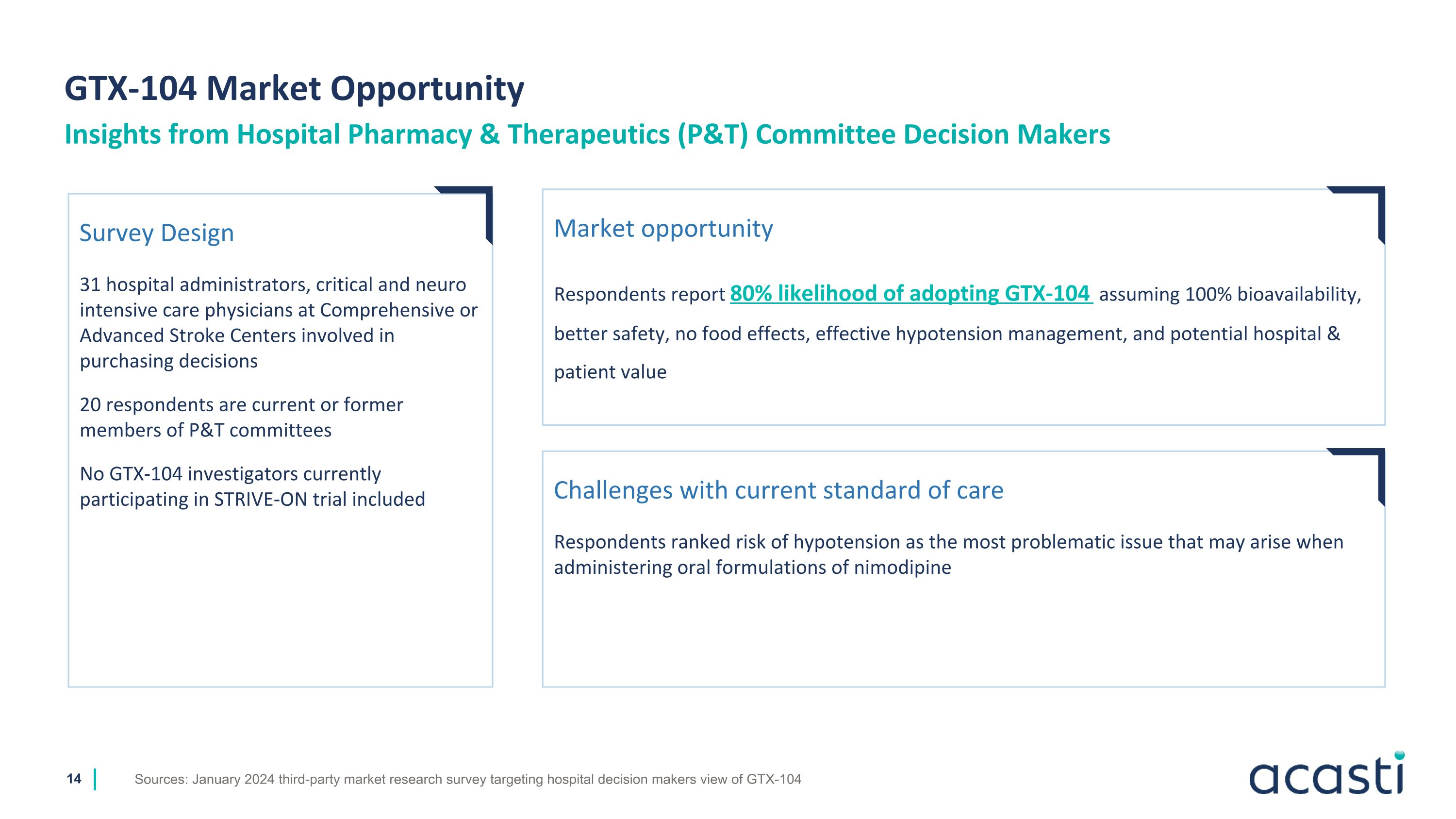
GTX-104 Market Opportunity Sources: January 2024 third-party market research survey targeting hospital decision makers view of GTX-104 Insights from Hospital Pharmacy & Therapeutics (P&T) Committee Decision Makers Survey Design 31 hospital administrators, critical and neuro intensive care physicians at Comprehensive or Advanced Stroke Centers involved in purchasing decisions 20 respondents are current or former members of P&T committees No GTX-104 investigators currently participating in STRIVE-ON trial included Market opportunity Respondents report 80% likelihood of adopting GTX-104 assuming 100% bioavailability, better safety, no food effects, effective hypotension management, and potential hospital & patient value Challenges with current standard of care Respondents ranked risk of hypotension as the most problematic issue that may arise when administering oral formulations of nimodipine

Strong and multi-layered intellectual property protection strategy Intellectual Property Portfolio GTX-104 received orphan drug status designation from the FDA Potential 7 years of marketing exclusivity in US upon NDA approval Strong US and international patent estate Consists primarily of composition and method-of-use patents to extend exclusivity beyond what is granted through the orphan drug designation. Multiple patents granted worldwide, including five patents in the US Long patent shelf-life First patent filed life 2037 Newest patent life 2042 Continue building our patent portfolio by filing for patent protection on new developments

Experienced Leadership Team Proven drug development and commercialization expertise Carrie D'Andrea VP Clinical Operations Loch Macdonald, MD, PhD Chief Medical Officer Prashant Kohli Chief Executive Officer Amresh Kumar, PhD VP Program Management Brian Ford, CPA-CA CFO Robert J. DelAversano Principal Financial Officer and Principal Accounting Officer
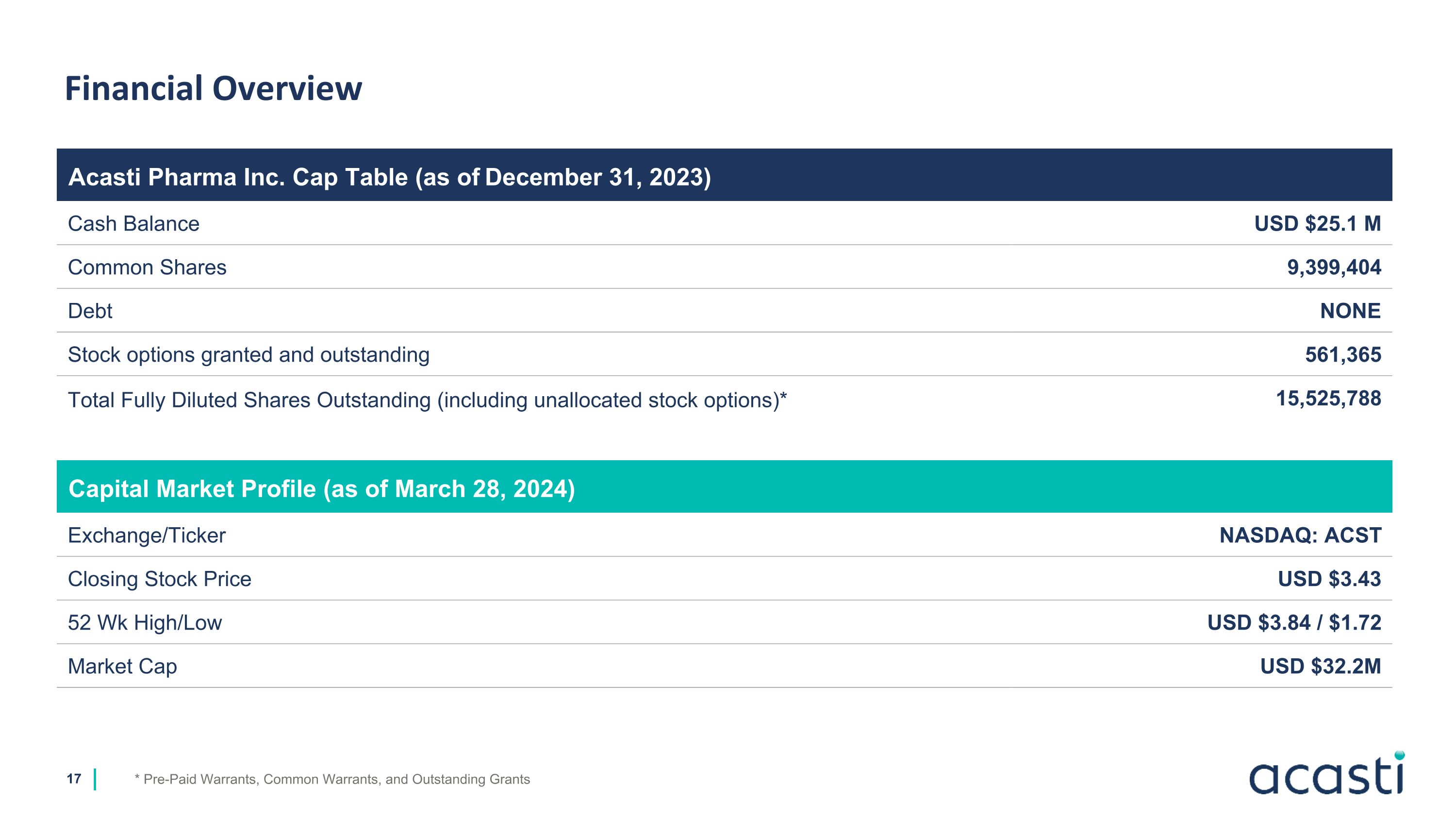
Financial Overview * Pre-Paid Warrants, Common Warrants, and Outstanding Grants Acasti Pharma Inc. Cap Table (as of December 31, 2023) Cash Balance USD $25.1 M Common Shares 9,399,404 Debt NONE Stock options granted and outstanding 561,365 Total Fully Diluted Shares Outstanding (including unallocated stock options)* 15,525,788 Capital Market Profile (as of March 28, 2024) Exchange/Ticker NASDAQ: ACST Closing Stock Price USD $3.43 52 Wk High/Low USD $3.84 / $1.72 Market Cap USD $32.2M

Acasti Contact: Prashant Kohli Chief Executive Officer Email: p.kohli@acasti.com Website: www.acasti.com Investor Contact: Mike Moyer LifeSci Advisors Email: mmoyer@lifescieadvisors.com